

The bond will be formed from a large lobe. Each hybrid orbital has two lobes, one is longer, and the other is smaller. The number of hybrid orbitals formed is always equivalent to the number of atomic orbitals that may take part in the process of hybridization.
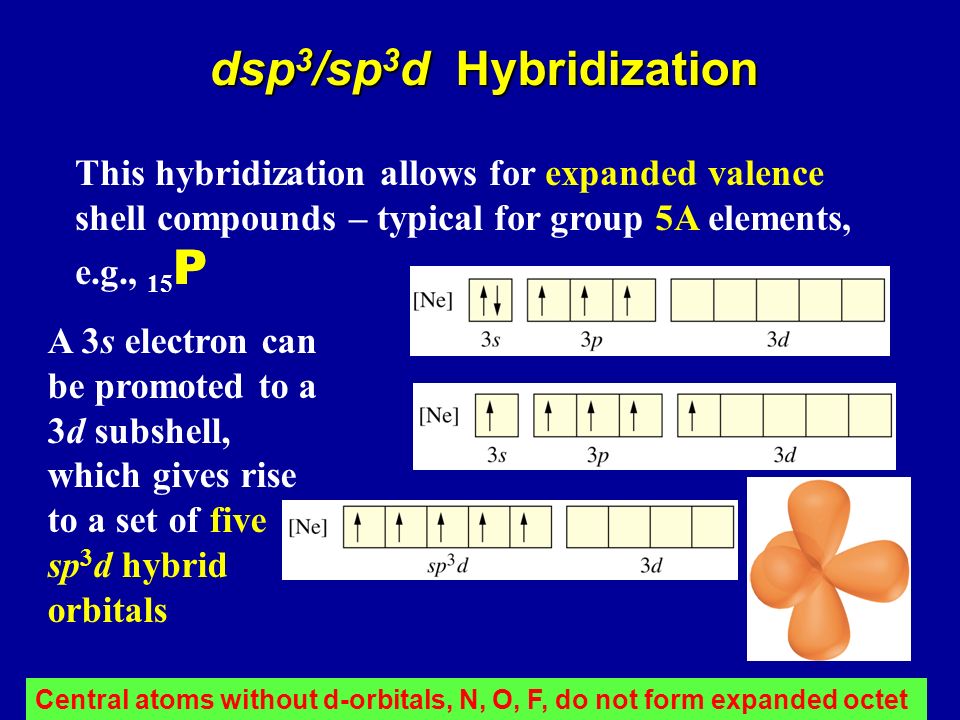
Therefore in hybridization full-filled, half-filled, and empty orbitals may take part. Hybridization is a process of mixing of orbitals and not electrons. Here in the first bond from the left side p-sp hybridization is present, and in the second bond sp-p hybridization is present, so the bond strength of both the bonds will be equal. Now consider sp hybridization in Beryllium Dichloride, Cl-Be-Cl. This problem can be overcome if the hybridization of s and p orbital occurs. Practically bond strength and distance of both the Be-Cl bonds are the same. If it is formed without hybridization then both the Be-Cl bonds should have different parameters and p-p bond strength > s-p bond strength. Consider an example of the Be Cl₂ compound. These new orbitals are called hybrid orbitals and the phenomenon is called hybridization. It can also be defined as the mixing of different shapes and atomic orbitals at approximately equal energy and redistribution of energy to form new orbitals, of the same shape and the same energy. Hybridization was introduced by Pauling, to explain the equivalent nature of covalent bonds in a molecule.


 0 kommentar(er)
0 kommentar(er)
